
Comprehensive guide to ISO 20795-1 for dental base polymers: material types (Types 1–5), key requirements, flexural testing methods, FDA/EN compliance, water sorption/solubility limits, and lab QA pra...
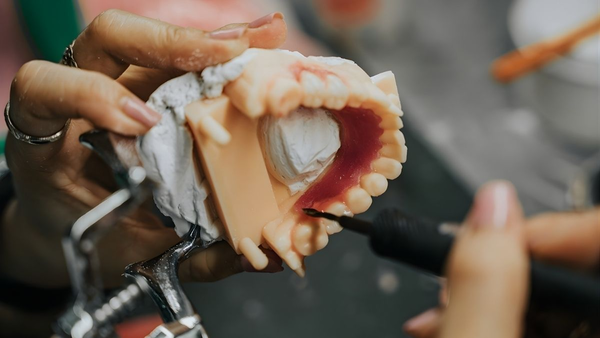
Ensure reliable dental outsourcing with ISO 9001/13485, FDA 21 CFR 820, and MDR. Vendor qualification, traceability, QA protocols, and CAPA for consistent quality.

Concise, practical guide to Good Manufacturing Practices (GMP). Covers regulatory frameworks (21 CFR), quality systems, documentation, validation, inspections, data integrity (ALCOA+), and best practi...

Creating a safe work environment in dental labs is a human commitment to the technicians whose craftsmanship shapes every restoration delivered to patients. By adopting a safety framework, strengtheni...

Explore how NADL drives innovation, sets industry standards, and supports dental labs like XDENT LAB in adapting to modern advancements in dental technology.

ISO 15189:2022 is a globally recognized standard that specifies requirements for quality and competence in medical laboratories, including dental labs involved in diagnostic testing and laboratory ser...

Vietnam has rapidly emerged as a global hub for dental manufacturing, driven by its competitive pricing, skilled workforce, and adherence to international quality standards. At the heart of this succe...

In today’s interconnected world, dental practices often rely on international dental laboratories for cost-effective solutions and high-quality products. While these partnerships offer significant adv...

ISO certification is a vital benchmark for dental laboratories aiming to establish credibility, ensure product quality, and expand into international markets. For Vietnamese dental labs, achieving ISO...

Vietnam has emerged as a key player in global dental production, providing high-quality dental materials and services to international markets. With its growing reputation, the country has established...

ISO 13485:2016 is the globally recognized standard for quality management systems (QMS) specific to the medical devices industry. For dental laboratories in Vietnam, achieving ISO 13485 certification...

The U.S. Food and Drug Administration (FDA) plays a critical role in regulating the safety, efficacy, and quality of medical devices, including dental products, imported into the United States. For Vi...


