Learn how fluoride‑releasing resin sealants work—triphasic release, recharge, isolation techniques, and retention metrics—to boost prevention and long‑term results.
Table of contents [Show]
- Introduction to Fluoride-Releasing Resin Sealants
- Composition and Material Science
- Clinical Applications and Techniques
- Fluoride Release Kinetics and Dynamics
- Comparative Effectiveness Studies
- Mechanisms of Caries Prevention
- Clinical Indications and Patient Selection
- Material Properties and Performance
- Long-Term Clinical Outcomes
- Recharge Capability and Fluoride Uptake
- Cost-Effectiveness Analysis
- Future Developments and Innovations
- Clinical Guidelines and Recommendations
- XDENT LAB Perspective
- Conclusion
Introduction to Fluoride-Releasing Resin Sealants
Fluoride-releasing resin sealants combine the mechanical barrier of conventional resin sealants with the therapeutic benefit of fluoride ion release. By sealing pits and fissures on primary and permanent teeth, they reduce plaque accumulation and support remineralization. Evidence shows fissure sealants can prevent caries with a success rate around 61% over five years, and newer materials are engineered to enhance retention, fluoride kinetics, and clinical reliability.
Composition and Material Science
Understanding formulation drives clinical performance, fluoride kinetics, and handling.
Chemical Composition of Fluoride-Releasing Resin Sealants
Base resin components: Bis-GMA (matrix), TEGDMA (diluent), UDMA (alternative base), HEMA (hydrophilic monomer), photoinitiators (camphorquinone/amine).
Fluoride-releasing components: Sodium fluoride particles (direct source), fluoroaluminosilicate glass (ion-releasing filler), ytterbium trifluoride (radiopaque fluoride source), calcium fluoride (sustained release), fluoride-containing polymers (controlled release systems).
Fluoride Release Mechanisms
Surface dissolution: Initial burst release from surface-bound fluoride; pH and water-mediated dissolution; early cariostatic effect.
Diffusion-controlled release: Fluoride migrates through the resin matrix via concentration gradients; enables sustained release and recharge after topical fluoride exposure.
Erosion-based release: Gradual polymer wear and filler exposure contribute to long-term, low-level release.
Clinical Applications and Techniques
Meticulous technique and isolation determine retention, microleakage, and fluoride effectiveness.
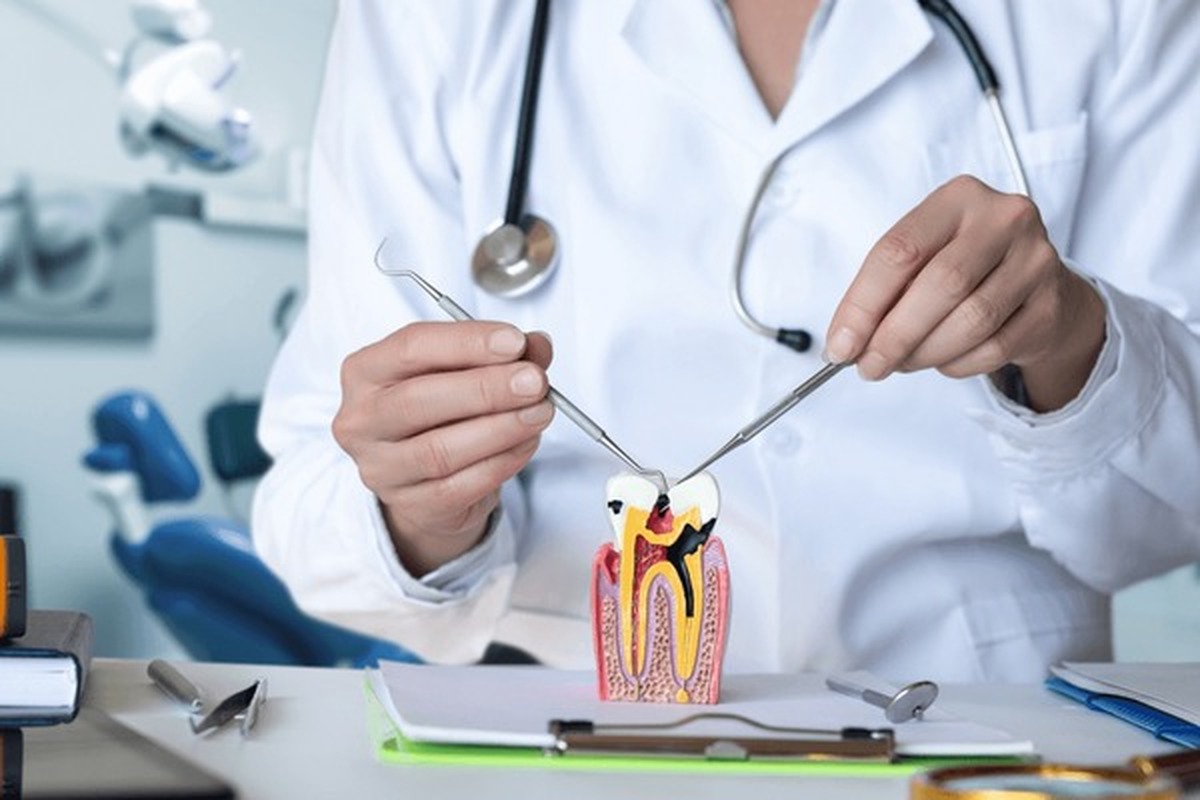
Application Protocols
Tooth preparation: Prophylaxis with pumice, thorough rinse, complete drying, fissure evaluation, caries detection.
Acid etching: 37% phosphoric acid for 15–30 seconds; rinse 20 seconds; dry to chalky enamel appearance.
Sealant placement: Controlled, bubble-free application; complete fissure coverage; avoid overfilling; allow proper flow.
Polymerization: Light cure 20–40 seconds; multiple exposures for deep fissures; verify light intensity and angulation.
Finishing: Check occlusion; remove excess; evaluate surface; optional fluoride varnish; provide patient instructions.
Isolation Methods Impact
Rubber dam: Gold standard moisture control; highest retention; technique- and time-sensitive.
Cotton rolls: Practical and cost-effective; adequate for many cases; contamination risk; requires assistant.
Dry field systems: Continuous suction devices; better than cotton rolls; slightly below rubber dam; good compromise.
Fluoride Release Kinetics and Dynamics
Sealants typically show a triphasic release profile with recharge capability.
Release Profiles
Initial burst (0–24 h): High fluoride concentration (≈5–20 ppm in saliva); rapid decline; early cariostatic effect.
Sustained phase (days–months): Lower steady levels (≈0.5–2 ppm); diffusion-controlled; supports remineralization over time.
Recharge phase: Uptake from topical fluoride (varnish, gel, rinses, high-fluoride toothpaste) renews release in cyclical patterns.
Factors Affecting Fluoride Release
pH: Acidic conditions increase release; materials may exhibit smart responses under cariogenic challenges.
Temperature: Higher temperatures can increase release; thermal cycling impacts kinetics and stability.
Mechanical stress: Occlusal forces and wear expose fresh surfaces, modestly enhancing long-term release.
Comparative Effectiveness Studies
Retention, caries prevention, and handling vary by material class and clinical conditions.
Clinical Performance Metrics
Retention at 24 months: Fluoride resin 75–85%; traditional resin 80–90%; glass ionomer 50–70%; bioactive materials 70–80%.
Partial retention: Fluoride resin 10–15%; traditional resin 5–10%; glass ionomer 20–30%; bioactive 15–20%.
Caries prevention: Overall 85–95%; secondary caries reduction ≈30–40%; adjacent surface protection documented; significant remineralization evidence.
Material Comparisons
Fluoride resin vs traditional resin: Slightly lower retention (−5–10%), but enhanced caries prevention (+10–15%); similar handling; moderately higher cost (+20–30%); equivalent chairside time.
Fluoride resin vs glass ionomer: Higher retention (+20–30%); lower moisture sensitivity; less fluoride than GI but more sustained; superior wear resistance; higher technique sensitivity.
Mechanisms of Caries Prevention
Protection is multimodal—physical barrier, chemical action, and biological effects.
Multi-Modal Protection
Physical barrier: Fissure isolation; exclusion of bacteria and nutrients; biofilm disruption; mechanical protection against plaque retention.
Chemical protection: Fluoride release; pH buffering; remineralization promotion; antibacterial impacts; ion exchange at the enamel surface.
Biological effects: Inhibition of bacterial metabolism; biofilm modification; ecological pressure favoring less cariogenic species.
Remineralization Dynamics
Fluoride ion activity: Enamel incorporation; fluorapatite formation; crystal growth; defect repair; surface strengthening.
Subsurface effects: Lesion arrest; mineral deposition; pore reduction; increased hardness; inhibition of lesion progression.
Clinical Indications and Patient Selection
Risk-based selection maximizes cost-effectiveness and clinical impact.
Primary Indications
High caries risk: Prior caries, deep fissures, poor hygiene, cariogenic diet, relevant medical conditions.
Tooth conditions: Newly erupted molars; deep pits and fissures; stained fissures; incipient non-cavitated lesions; hypoplastic defects.
Age: First molars (6–7 years); second molars (12–13 years); selected primary molars; adults and special needs patients based on risk.
Contraindications and Limitations
Contraindications: Frank cavitation; inability to isolate; partially erupted teeth; allergy to components; proximal caries.
Relative: Shallow fissures; low caries risk; excellent hygiene; regular fluoride exposure; cost considerations.
Material Properties and Performance
Physical and optical properties influence handling, durability, and monitoring.
Physical Properties
Viscosity: Optimal flow ≈500–1000 cP for penetration without bubble formation; temperature-sensitive handling.
Polymerization shrinkage: ≈2–4% volume reduction; manage stress to protect marginal integrity and prevent gaps.
Wear resistance: Essential in occlusal contact areas; adequate thickness and filler selection improve durability.
Optical Properties
Color options: Clear (aesthetics), opaque white (monitoring), tinted (placement verification); focus on long-term color stability.
Translucency: Natural appearance, visibility of underlying tooth structure, aids monitoring for caries detection.
Long-Term Clinical Outcomes
Retention and protective benefits persist with maintenance and reapplication as needed.
Longitudinal Results
Five-year outcomes: Complete retention 60–70%; partial retention 15–20%; caries prevention 85–90%; reapplication 20–30%; demonstrated cost-effectiveness.
Ten-year follow-up: Retention 40–50%; continued protection documented; fluoride release still detectable; high patient satisfaction.
Failure Analysis
Complete loss: Poor initial retention; moisture contamination; inadequate etching; high occlusal wear; technique errors.
Partial loss: Marginal breakdown; central wear; peripheral retention; ongoing protection possible; requires monitoring.
Secondary caries: Marginal leakage; incomplete coverage; material degradation; patient-related factors; largely preventable with protocol adherence.
Recharge Capability and Fluoride Uptake
Recharge extends protective lifespan and supports risk-based maintenance.
Fluoride Recharge Mechanisms
Fluoride sources: Professional varnish/gel; high-fluoride toothpaste; daily rinses; dietary fluoride; tailored to patient risk.
Uptake kinetics: Concentration- and time-dependent; material-specific saturation; renewed release post-exposure.
Clinical Recharge Protocols
Professional care: Varnish quarterly; gels biannually; high concentration (≈22,600 ppm) with 4-minute contact where indicated.
Home care: 5000 ppm toothpaste for high-risk adults/teens; 225–900 ppm rinses; emphasize compliance and synergy with office applications.
Cost-Effectiveness Analysis
Preventive value compounds through avoided restorations and reduced chairtime.

Economic Evaluation
Initial costs: Material ≈$15–25 per tooth; application 15–20 minutes; minimal equipment; standard training; low overhead.
Long-term savings: Avoided restorations ≈$150–300 each; reduced treatment time; improved patient satisfaction and practice efficiency.
Cost-Benefit Ratios
Cost per prevented cavity: ≈$50–100.
ROI: ≈3:1 to 5:1; break-even ≈2–3 years; lifetime savings significant; positive quality-adjusted life years.
Future Developments and Innovations
Material science is trending toward responsive, bioactive, and simplified systems.
Emerging Technologies
Smart materials: pH-responsive release, bioactive fillers, self-healing resins, antibacterial agents, nanotechnology integration.
Enhanced delivery: Controlled-release polymers, multilayer architectures, targeted delivery, sustained profiles, combination therapies.
Research Directions
Improved retention mechanisms.
Optimized fluoride kinetics and recharge.
Biocompatibility and aesthetics enhancements.
Simplified application and isolation techniques.
Clinical Guidelines and Recommendations
Evidence-based protocols maximize protection and minimize failures.
Best Practice Guidelines
Patient selection: Use risk assessment tools; individual evaluation; discuss cost-benefit; obtain informed consent; schedule follow-ups.
Technique optimization: Prioritize moisture control; adhere to etching times; confirm curing intensity; verify coverage; document materials and lot numbers.
Professional Recommendations
Supported by major dental organizations for caries prevention in at-risk populations.
Strong evidence base and high recommendation grade when proper technique and follow-up are applied.
XDENT LAB Perspective
XDENT LAB delivers Lab-to-Lab full service from Vietnam with FDA and ISO alignment, certified technicians, and digital workflows. For sealant-related preventive programs, we prioritize documented materials, traceability, and quality assurance. Our compliance-first approach helps practices integrate fluoride-releasing resin sealants into risk-based hygiene protocols, improving patient outcomes while maintaining consistent quality.
Conclusion
Fluoride-releasing resin sealants offer dual-action protection: a physical barrier against plaque retention and sustained fluoride release to promote remineralization. With careful isolation, precise placement, and recharge-oriented maintenance, these materials deliver strong caries-preventive outcomes across age groups. Ongoing innovations in smart, bioactive chemistries and simplified application systems will further enhance retention, fluoride kinetics, and cost-effectiveness—making fluoride-releasing resin sealants a foundational tool in comprehensive caries prevention strategies.
XDENT LAB is an expert in Lab-to-Lab Full Service from Vietnam, with the signature services of Removable & Implant, meeting U.S. market standards – approved by FDA & ISO. Founded in 2017, XDENT LAB has grown from local root to global reach, scaling with 2 factories and over 100 employees.. Our state-of-the-art technology, certified technicians, and commitment to compliance make us the trusted choice for dental practices looking to ensure quality and consistency in their products.

Our commitments are:
100% FDA-Approved Materials.
Large-Scale Manufacturing, high volume, remake rate < 1%.
2~3 days in lab (*digital file).
Your cost savings 30%.
Uninterrupted Manufacturing 365 days a year.
Contact us today to establish a strategy to reduce operating costs.
--------❃--------
Vietnam Dental Laboratory - XDENT LAB
🏢 Factory 1: 95/6 Tran Van Kieu Street, Binh Phu Ward, Ho Chi Minh City, Vietnam
🏢 Factory 2: Kizuna 3 Industrial Park, Can Giuoc Commune, Tay Ninh Province, Vietnam
☎ Hotline: 0919 796 718 📰 Get detailed pricing
Share this post: