Acrylic resins, especially PMMA, have transformed denture fabrication with its strength, esthetics, and adaptability. This article explores the evolution of acrylic materials, highlighting their chemical structure, curing mechanisms, and diverse applications in dentistry. Discover why acrylic remains the gold standard for denture bases even in the digital era of prosthetic design.
Table of contents [Show]
Key Points
PMMA remains the gold standard denture base material due to its balance of strength, esthetics, and cost.
Different curing methods significantly affect mechanical properties and clinical performance.
Digital acrylic forms (CAD/CAM & 3D printing) reduce porosity and improve consistency.
Material selection should be based on indication, workflow, and patient needs.
Introduction
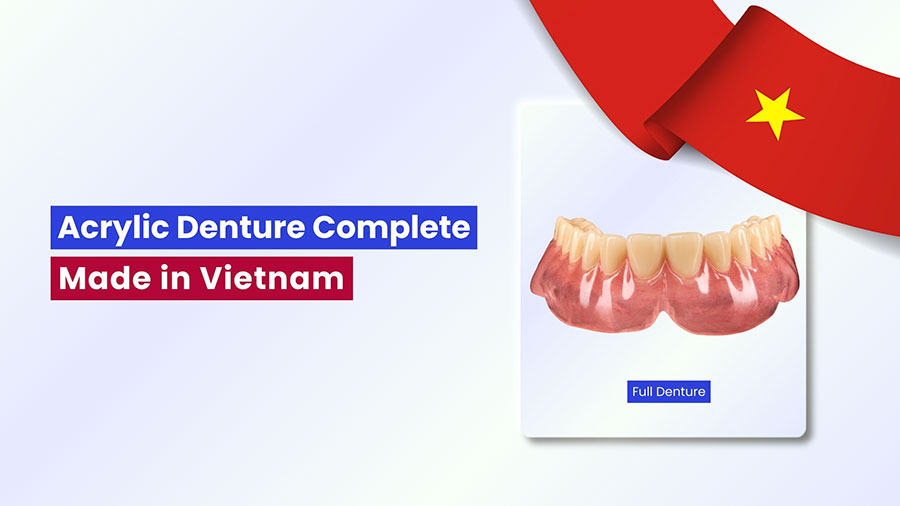
Although dental implants have become the gold standard for tooth replacement, removable dentures remain a vital treatment option, particularly for patients with systemic health conditions such as diabetes or cardiovascular disease, or those with severe jawbone resorption. For removable dentures to function effectively, the denture base plays a crucial role as it supports the artificial teeth, replaces lost soft tissues, and transmits masticatory forces to the underlying oral structures. Because it endures constant stress and must fit harmoniously within the oral environment, the selection of an appropriate denture base material has always been a key focus in prosthodontic development.
Over the past two centuries, dental science has explored a wide variety of materials for denture bases, including wood, ivory, metals, cellulose products, epoxy resins, polystyrene, polycarbonates, Bakelite, vinyl resins, nylon, and vulcanite. Each of these materials, however, presented significant drawbacks in terms of appearance, durability, or handling. For example, vulcanite, produced by heating natural rubber with sulfur, was the first material used for mass production of dentures but suffered from its dark, unesthetic color. Vinyl resins offered improved workability but exhibited poor fatigue and fracture resistance. Likewise, phenol-formaldehyde (Bakelite) and cellulose nitrates, introduced in the early 20th century, marked progress in synthetic polymers but were limited by processing difficulties, color instability, warpage, and unpleasant taste.
By 1937, these limitations led to the replacement of vulcanite by polymethyl methacrylate (PMMA), a material that would transform the field of prosthodontics. Since its introduction, acrylic resins have been used not only for denture bases but also for artificial teeth, denture repairs, impression trays, record bases, temporary crowns, bridge facings, and obturators for cleft palates. The arrival of PMMA marked a turning point in dental materials, offering a combination of mechanical strength, esthetic versatility, and cost efficiency. Unlike metal-based frameworks, acrylic resins provide natural translucency, precise color matching, and easy repairability, enhancing both function and appearance. What makes acrylic resins truly exceptional is its chemical versatility. Through different polymerization methods such as heat-, chemical-, light-, or microwave-activated processes, acrylic resins can adapt to various laboratory workflows without compromising quality. Despite some ongoing challenges such as fracture risk and residual monomer content, acrylic resins continue to dominate dental prosthetics due to its balance of durability, esthetics, and affordability.
Ideal Properties of a Denture Base Material
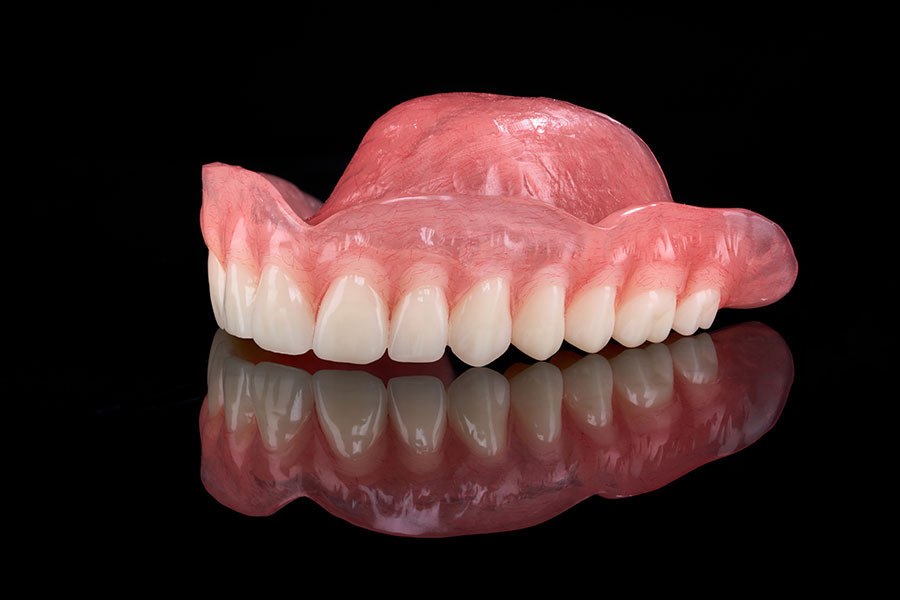
The widespread use of acrylic resins, particularly polymethyl methacrylate (PMMA), stems from their ability to meet most of the requirements expected of an ideal denture base material. Before understanding how acrylic resins achieve this balance, it is essential to define what characteristics a denture base must possess to function effectively within the oral environment.
A denture base operates under continuous mechanical stress, fluctuating temperatures, and constant exposure to saliva, food, and microorganisms. To ensure long-term clinical success and patient comfort, the material must not only be strong and stable but also biocompatible and aesthetically pleasing. An ideal denture base material should therefore exhibit the following key properties:
Biological:
Biocompatible, non-toxic, non-irritant, and non-carcinogenic to oral tissues, ensuring long-term safety and patient comfort.
Chemical:
Insoluble and chemically inert in saliva and dietary fluids.
Resistant to fluid absorption and dimensional changes.
Able to form strong chemical bonds with artificial teeth and soft liners.
Mechanical:
High modulus of elasticity and adequate rigidity to withstand masticatory forces.
Resilient enough to absorb occlusal stresses and protect soft tissues.
High fatigue and impact strength for daily functional loads.
Dimensional stability, low density, and good abrasion resistance, especially important for maxillary dentures.
Thermal:
Good thermal conductivity to transmit natural temperature sensations.
A coefficient of thermal expansion (CTE) compatible with artificial teeth.
A high softening temperature above 100 °C to maintain stability during processing and use.
Esthetic:
Sufficient translucency and capability of pigmentation to mimic natural oral tissues.
Practical and Other Properties:
Easy to manipulate, repair, and clean.
Cost-effective and durable with long-term property retention.
Radiopaque for safety and traceability if accidentally ingested.
Long shelf life and stable performance under various environmental conditions.
While many materials have been proposed to fulfill these ideal requirements, few have successfully achieved the right balance between functionality, aesthetics, and biocompatibility. Among them, polymethyl methacrylate (PMMA) stands out as the material that most closely meets these criteria. Thanks to its favorable combination of mechanical strength, dimensional stability, and esthetic adaptability, PMMA has become the benchmark for denture base fabrication. Its widespread adoption represents the culmination of decades of innovation in prosthodontic materials, and sets the stage for understanding how acrylic resins have evolved to meet clinical and laboratory demands in modern dentistry.
Acrylic Denture Base Materials
Development of Denture Base Materials
Having established the ideal requirements of a denture base, it is important to understand how material development evolved toward meeting those standards. From 1840 to 1940, various natural and synthetic substances were explored for denture base fabrication, but none fully satisfied the demands for durability, esthetics, and biocompatibility. Mechanical retention through clasps and undercuts was the primary means of securing dentures until the introduction of acrylic resin revolutionized the field.
Vulcanite: The first material used for mass production of dentures, vulcanite was produced by heating natural rubber with sulfur. While it enabled affordable denture manufacturing, its major drawbacks included dark color, porosity, and poor hygiene due to microbial accumulation within the base.
Celluloid: Developed by plasticizing cellulose nitrate with camphor, celluloid offered better esthetics but suffered from several issues such as rapid discoloration, high water absorption, a persistent camphor taste, and poor repairability. These disadvantages limited its clinical use.
Bakelite: The introduction of phenol-formaldehyde (Bakelite) in the early 20th century represented another step forward. However, difficulties in processing, instability of color, unpleasant taste, and lack of reparability kept Bakelite from becoming a desirable denture base material. By 1937, Bakelite and vulcanite were largely replaced by polymethyl methacrylate (PMMA).
Polyvinyl Chloride (PVC): Although still used as a denture lining material and in athletic mouthguards, PVC proved unsuitable for full denture bases due to low fracture resistance, loss of plasticizer over time, and difficult polishing, leading to poor hygiene and mucosal irritation.
Polymethyl Methacrylate (PMMA): Acrylic resin was first synthesized in 1900 by the German chemist Rohn and has been widely applied in medicine and dentistry since 1940 following its patent by Kulzer. The polymer is produced by polymerizing methyl methacrylate (MMA) in an aqueous environment, resulting in small polymethyl methacrylate (PMMA) spheres approximately 40–100 μm in diameter. A key milestone in industrial PMMA production came in the early 1930s, when Crawford introduced a rapid synthesis method using commonly available chemicals such as acetone, hydrocyanic acid, methanol, and sulfuric acid. This advancement not only accelerated large-scale polymer production but also made acrylic resin more accessible and consistent in quality, laying the foundation for its adoption in medical and dental applications.
With improved availability and superior material properties, PMMA quickly became the dominant choice for denture bases and remains the gold standard today. In prosthodontics, acrylic resins are also used for artificial teeth, temporary crowns, impression trays, record bases, and obturators for cleft palates. Fully polymerized PMMA is transparent and can be tinted to match gingival color. However, incomplete polymerization may leave residual monomer content (0.2–0.5% in heat-cured and 2–5% in self-cured acrylics), potentially causing mucosal irritation or allergic reactions. PMMA is insoluble in water but soluble in aromatic hydrocarbons, ketones, and esters. Alcohol acts as a plasticizer, lowering its glass transition temperature (Tg); thus, alcohol-based cleaning or storage solutions should be avoided.
While PMMA is favored for its low cost, lightweight, easy handling, high esthetics, and repairability, it also exhibits several weaknesses: low thermal conductivity, relatively low mechanical strength, brittleness, and dimensional changes due to water absorption. These limitations have motivated ongoing research into improving PMMA’s properties through compositional modification or reinforcement with advanced materials such as:
Fibers: aramid, carbon/graphite, polyethylene, and glass fibers
Metallic fillers: aluminum or titanium microparticles
Nanoparticles (NPs): silica, zirconia, or titania NPs to enhance strength and reduce water sorption
Another challenge with acrylic resins is radiolucency, making them difficult to detect if swallowed. Incorporating 10–15% of bismuth or uranyl salts improves radiopacity but may also increase water sorption and flexibility. Achieving optimal radiodensity without compromising mechanical and esthetic performance remains a research focus.
Chemical Structure and Polymerization Mechanism of Acrylic Resins
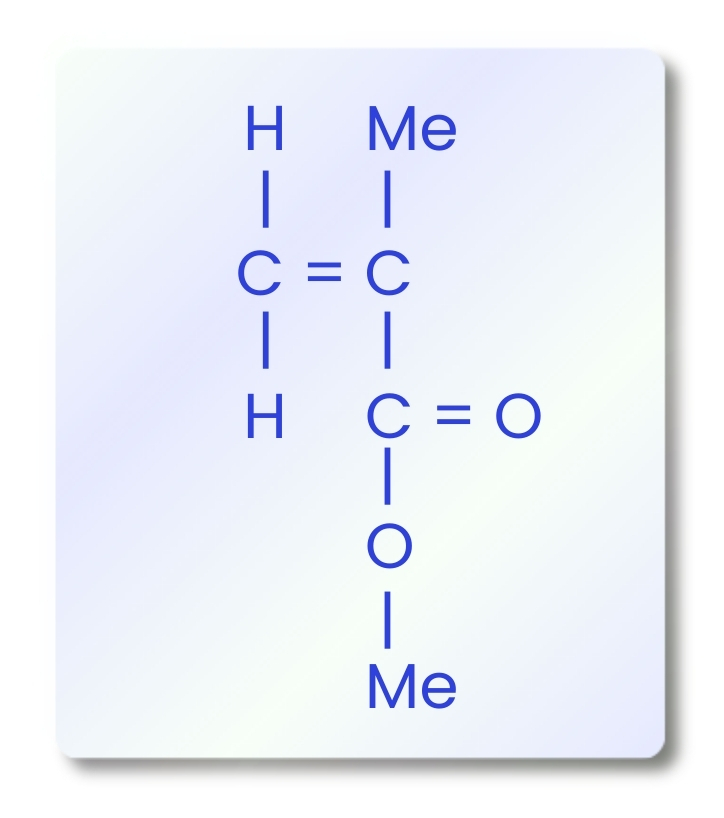
To better understand the properties that make acrylic an indispensable denture base material, it is necessary to examine its chemical structure and polymerization behavior. The primary component of acrylic resins is polymethyl methacrylate (PMMA), a polymer formed through the free-radical addition polymerization of methyl methacrylate (MMA) monomers. The molecular structure of MMA contains a carbon–carbon double bond, which enables polymer chains to grow through the successive addition of activated monomers.
This polymerization process proceeds through main stages: activation, initiation, propagation, and termination. Depending on how activation occurs, acrylic resins are classified into heat-cured, cold-cured, light-activated, and microwave-cured types.
Activation and Initiation: The process begins with the generation of free radicals, which can be initiated by heat, light, chemical agents, or peroxides. These free radicals attack the double bond of MMA, forming a reactive site that starts the chain reaction.
Propagation: During this stage, successive MMA monomers rapidly attach to the growing polymer chain, transferring the free electron to the end of the chain and extending the polymer backbone.
Termination: The reaction ends when two free radicals combine or when an inhibitor reacts with the active radical site, halting further chain growth. Substances such as hydroquinone, eugenol, or oxygen can inhibit or slow down polymerization by neutralizing free radicals.
The balance between initiation and termination determines the molecular weight and mechanical performance of the final PMMA product. Lower initiation rates can delay polymerization, while excessive termination can reduce chain length and weaken the material. To enhance shelf life and stability, small quantities of hydroquinone are added to the MMA monomer as a stabilizer, preventing premature polymerization during storage. Through controlled polymerization, PMMA achieves an optimal molecular structure that offers dimensional stability, optical clarity, and balanced mechanical strength, qualities that have made it the most widely used acrylic resin in removable prosthodontics.
Curing Mechanisms of Acrylic Materials

Understanding the polymerization chemistry of acrylic resins provides the foundation for how these materials are processed in clinical and laboratory settings. In practice, the polymerization or curing of acrylics can be achieved through several activation methods, each influencing the material’s properties, handling characteristics, and clinical performance. These methods determine not only how effectively the resin polymerizes but also the strength, stability, and accuracy of the final denture base.
Heat-Cured Acrylic Resins
Among all denture base materials, heat-cured acrylics remain the gold standard, valued for their mechanical stability, esthetic results, and proven clinical longevity in everyday prosthodontic practice. Heat-cured acrylics remain the most common material for denture base fabrication thanks to their strength, reliability, and long-term performance. These resins are typically supplied as a powder–liquid system: the powder contains pre-polymerized PMMA beads, pigments, and an initiator (benzoyl peroxide), while the liquid includes methyl methacrylate (MMA) with a small amount of inhibitor and cross-linking agent. When the material is processed under heat, the initiator activates and converts the MMA into solid PMMA. The result is a denture base that is strong, durable, and highly esthetic, which explains why heat-cured PMMA remains the benchmark for removable dentures worldwide.
However, during polymerization, minor shrinkage may occur due to heat and stress release, which can slightly affect the fit. Despite this, its superior mechanical stability and color retention continue to make it the preferred material in both clinical and laboratory settings. A faster alternative, known as rapid heat-polymerized acrylic, combines heat and chemical initiators to complete polymerization within about 20 minutes in boiling water. This offers a practical option for labs seeking to save processing time without compromising on strength or quality.
Advantages
Excellent mechanical strength and dimensional stability
Superior esthetics and long-term color retention
High degree of polymerization, resulting in fewer residual monomers
Proven biocompatibility and clinical reliability
Cost-effective and widely available
Limitations
Polymerization shrinkage may reduce adaptation accuracy
Requires controlled heating and de-flasking to prevent distortion
Longer curing time compared to other resin systems
Not easily adjustable once processed
Examples:
Lucitone 199® (Dentsply Sirona, USA)
Meliodent® (Kulzer, Germany)
ProBase Hot® (Ivoclar Vivadent, Liechtenstein)
QC 20® (Dentsply, UK)
Acrosun® (GC Corporation, Japan)
Acropars® (Marlic Medical Industries, Iran).
Cold-Cured Acrylic Resins
Cold-cured acrylic resins (also known as self-cured or autopolymerizing acrylics) share a similar chemical composition with heat-cured resins; however, their polymerization is initiated chemically rather than thermally. A tertiary amine such as dimethyl-p-toluidine or sulfinic acid, acts as the activator, starting the polymerization process at room temperature. Because polymerization occurs without external heating, the reaction is generally less complete, leading to polymers with lower molecular weight, reduced strength, and higher residual monomer content compared to heat-cured PMMA. The residual monomers can irritate oral tissues and compromise color stability over time. Another limitation is their susceptibility to creep and deformation. With a glass transition temperature typically between 75°C–80°C, cold-cured acrylics are softer and more prone to warping under stress or heat. Surface polymerization can also be inhibited by oxygen, reducing conversion efficiency; this can be mitigated by curing the material in water to improve polymerization.
Despite these disadvantages, cold-cured acrylics remain indispensable in prosthodontics due to their speed, convenience, and ease of use. They are widely used for repairs, relines, temporary denture bases, and custom trays, requiring no specialized equipment and curing rapidly at room temperature, ideal for chairside or laboratory quick-turnaround applications.
Advantages
Fast polymerization at room temperature
No need for special curing equipment
Useful for relining, repairing, or fabricating temporary dentures
Better dimensional accuracy due to reduced polymerization shrinkage
Limitations
Lower mechanical strength and hardness compared to heat-cured PMMA
Higher residual monomer content may cause irritation or odor
Poor color stability and higher susceptibility to deformation
Shorter service life, suitable mainly for temporary or repair work
Examples:
Duralay® (Reliance Dental Mfg Co., USA)
Jet Denture Repair Resin® (Lang Dental, USA)
ProBase Cold® (Ivoclar Vivadent, Liechtenstein)
Palapress Vario® (Kulzer, Germany)
Tokuso Cure Fast® (Tokuyama Dental, Japan).
Visible Light–Cured Acrylic Resins
As dentistry advanced into the era of precision and efficiency, the need for faster, cleaner, and more controlled curing systems led to the development of visible light–cured (VLC) acrylic resins. Representing a newer generation of denture base materials, these resins polymerize under blue light at wavelengths between 400–500 nm. Unlike traditional PMMA-based resins, VLC systems typically employ urethane dimethacrylate (UDMA) as their main matrix, reinforced with acrylic beads and colloidal silica to regulate viscosity and improve handling. A photoinitiator, commonly camphorquinone, triggers polymerization upon exposure to visible light in a dedicated curing chamber. Mechanically, VLC resins exhibit comparable or slightly lower strength than conventional PMMA; however, they offer several practical advantages. Their polymerization is highly controlled and complete, resulting in low residual monomer content, minimal porosity, and excellent color stability. The resulting denture bases also demonstrate reliable adhesion to other acrylic materials, making them ideal for repairs, relining, custom trays, and partial denture applications, rather than for complete denture bases.
Nevertheless, visible light–cured materials can be brittle and technique-sensitive, requiring specialized light-curing equipment and precise exposure conditions. Additionally, their limited translucency restricts their use in highly aesthetic areas.
Advantages
Free of methyl methacrylate (non-toxic formulation)
Reduced porosity and improved dimensional accuracy
Fast and clean polymerization without residual compounds
Excellent surface finish and low bacterial adherence
Convenient for repairs, relines, and short-term use
Limitations
More brittle than conventional PMMA
Requires specific light-curing units (higher equipment cost)
Limited translucency and esthetic range
Technique-sensitive — polymerization depends on precise exposure and handling
Examples:
Triad VLC® (Dentsply Sirona, USA)
Eclipse® Light Cure Resin System (Dentsply Sirona, USA)
Lightplast Base® (Dreve-Dentamid GmbH, Germany)
QuickLine® (GC America, USA).
Microwave-Cured Acrylic Resins
As dental laboratories continued to seek faster and more energy-efficient processing methods, microwave-cured acrylic resins emerged in 1968 as an innovative alternative to conventional heat-polymerized systems. Instead of relying on prolonged hot-water curing cycles, polymerization in this technique is achieved through microwave energy, which rapidly activates the initiator molecules within the acrylic material. Because microwaves cannot penetrate metal, this process requires specialized nonmetallic (plastic) flasks and dedicated microwave-curing units. Although the initial equipment cost is higher, the technique dramatically reduces curing time as short as three minutes, greatly improving workflow efficiency and laboratory productivity.
Numerous studies have shown that dentures fabricated by microwave polymerization exhibit comparable mechanical strength and adaptation to those produced by traditional heat-cured PMMA. Additionally, they often show lower residual monomer content and enhanced color stability, making this approach especially attractive for high-volume dental laboratories aiming to accelerate production without sacrificing quality.
However, microwave polymerization is not without drawbacks. The bond strength between the denture base and acrylic teeth is often weaker than in heat-cured materials, and porosity may occur if heat becomes trapped within the nonmetallic flasks during curing. Moreover, plastic flasks and polycarbonate bolts are more fragile and tend to degrade over repeated cycles of pressure and heat exposure.
Advantages
Extremely short curing cycle (≈3 minutes)
Excellent dimensional accuracy and fit
Reduced residual monomer and improved color stability
Fewer fractures in both artificial teeth and resin bases
Ideal for fast production workflows in high-volume labs
Limitations
Weaker bond to denture teeth compared to heat-cured PMMA
Possible porosity from heat accumulation in plastic flasks
Specialized plastic flasks and microwave units increase cost
Equipment components wear out after repeated use under pressure
Examples:
Acron MC® (GC Corporation, Japan)
Acron-MC HI® (GC Corporation, Japan)
Onda-Cryl® (Artig Dental, Brazil)
Meliodent Microwave® (Kulzer, Germany).
Pour-Type (Fluid) Acrylic Resins
Following the development of microwave-curing technology, another notable advancement in acrylic processing was the introduction of pour-type, or fluid, acrylic resins. These materials are a subtype of cold-cured resins distinguished by their high flowability and ability to adapt precisely to mold details. After mixing, the resin forms a low-viscosity slurry that can be poured directly into agar hydrocolloid, silicone, or modified plaster molds, eliminating the need for conventional packing pressure. The smaller polymer particle size of pour-type resins enhances their flow characteristics, ensuring excellent mold adaptation. During fabrication, the material can be centrifugally cast or injected into the mold, promoting dimensional accuracy and minimizing air entrapment. This simplified workflow reduces manual labor and eliminates the need for specialized curing equipment, making it an attractive option for small to mid-sized laboratories seeking efficiency and cost-effectiveness.
However, pour-type acrylics present certain limitations. Their mechanical strength is generally lower than both heat- and cold-cured resins, and they exhibit higher solubility and residual monomer content, which may compromise long-term durability and patient comfort. For this reason, they are best suited for temporary or low-stress prosthetic applications, where ease of fabrication and affordability are prioritized over mechanical performance.
Advantages
Simplified and low-cost processing technique
Excellent flow and mold adaptation
Good dimensional stability with minimal shrinkage
No need for pressure or heat curing equipment
Limitations
Lower mechanical strength and fracture resistance
Higher solubility and residual monomer levels
Limited long-term performance under masticatory stress
Primarily suitable for temporary or short-term dentures
Examples:
SR Ivocap Pour® (Ivoclar Vivadent, Liechtenstein)
Lucitone Pourable® (Dentsply Sirona, USA)
Pour’n Cure® (Lang Dental, USA).
Commercial Forms and Composition of Acrylic Resins
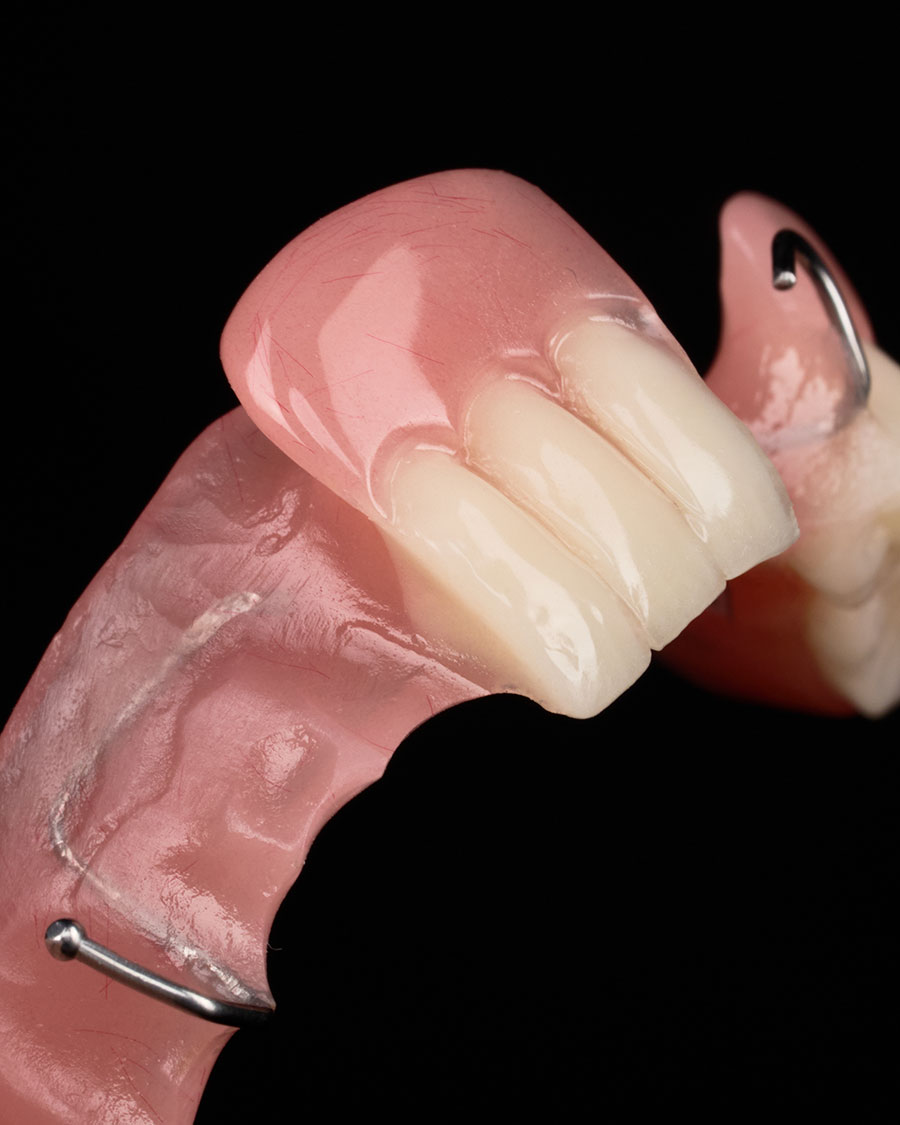
With the advancement of CAD/CAM and 3D printing technologies, acrylic resins are no longer limited to traditional powder–liquid systems. They now exist in multiple commercial forms. Each optimized for specific fabrication methods, levels of precision, and production workflows. The six most common commercial forms used in modern dental laboratories include:
Powder–Liquid System
Description: The conventional and most widely used form, consisting of PMMA polymer powder and MMA monomer liquid, suitable for heat-cured or cold-cured processes.
Applications: Removable dentures, relines, and denture repairs.
Examples:
Lucitone 199® (Dentsply Sirona, USA)
Acralyn-H® (Asian Acrylates, India)
Triplex Hot® (Ivoclar Vivadent, Liechtenstein).
Gel or Paste Form
Description: Acrylics presented as ready-to-use viscous gels or pastes, often light-curable (photo-polymerized) using UV or visible light.
Applications: Chairside relining, temporary restorations, and small denture repairs.
Examples:
Triad Gel® (Dentsply Sirona, USA)
QuickLine® (GC America, USA)
Lightplast Base® (Dreve, Germany).
CAD/CAM PMMA Block or Disc
Description: Fully polymerized PMMA blocks or discs designed for subtractive manufacturing using CAD/CAM milling systems. Industrial polymerization under controlled conditions ensures high molecular uniformity, resulting in superior fit, esthetics, and dimensional stability compared with conventionally processed materials. These pre-polymerized discs reduce internal porosity and eliminate polymerization shrinkage, providing consistent and precise results across digital workflows.
Applications: Milled denture bases, provisional crowns and bridges, and hybrid prosthetic frameworks.
Examples:
Ivotion® Disc (Ivoclar Vivadent, Liechtenstein)
Juvora® PMMA Disc (Juvora Ltd., UK)
Vita CAD-Temp® (Vita Zahnfabrik, Germany).
PMMA Pellets or Granules
Description: Small, rigid PMMA beads formulated for injection molding and industrial 3D printing applications. These pre-polymerized pellets enable high-pressure, precision fabrication with uniform density and minimal porosity. Compared with traditional powder–liquid systems, PMMA granules provide better process control, improved consistency, and enhanced mechanical properties, making them ideal for large-scale or automated denture production.
Applications: Injection-molded denture bases, orthodontic appliances, and night guards.
Examples:
ProBase Hot Injection Pellets® (Ivoclar Vivadent, Liechtenstein)
Vertex ThermoJect® (Vertex-Dental, Netherlands)
Acrytens® (Pressing Dental, Italy).
3D Printing Liquid Resin
Description: Photopolymerizable liquid acrylic or methacrylate-based resins designed for additive manufacturing (3D printing) via UV or laser curing. Unlike subtractive CAD/CAM milling, these resins solidify layer by layer, enabling the direct fabrication of complex and highly customized prosthetic structures. Controlled polymerization under digital parameters ensures high precision, reduced material waste, and improved reproducibility.
Applications: 3D-printed denture bases, provisional restorations, and custom trays.
Examples:
NextDent Denture 3D+® (3D Systems, USA)
Lucitone Digital Print™ 3D Resin (Dentsply Sirona, USA)
Asiga DentaBASE® (Asiga, Australia).
PMMA Sheets or Rods
Description: Pre-polymerized solid acrylic materials supplied in sheet or rod form, suitable for pressing, thermoforming, or manual shaping. Their high clarity, durability, and ease of machining make them suitable for both esthetic and protective dental applications, as well as broader medical use.
Applications: Occlusal splints, clear aligners, esthetic veneers, and medical-grade prosthetics.
Examples:
Orthocryl® Sheets (Dreve, Germany)
Paladur® Rods (Kulzer, Germany)
Acrycast® PMMA Sheets (Plaskolite, USA).
Each commercial form represents a unique balance between cost, precision, and convenience. Traditional powder–liquid systems remain the most economical choice for conventional dentures, while CAD/CAM PMMA discs and 3D-printable resins define the digital evolution of prosthetic manufacturing.
Conclusion
Acrylic resin has stood the test of time as one of the most versatile and reliable materials in modern prosthodontics. From its early use as a heat-cured powder–liquid system to today’s digitally engineered CAD/CAM blocks and 3D-printable resins, acrylic continues to evolve to meet the functional and aesthetic needs of both clinicians and patients.
Its unique combination of biocompatibility, esthetics, and ease of processing ensures that PMMA remains a cornerstone of denture base fabrication, even as new thermoplastics and hybrid polymers emerge. While challenges such as fracture resistance, residual monomer, and thermal conductivity persist, ongoing innovations in polymer chemistry and processing technology continue to refine acrylic’s performance. For dental laboratories, understanding the properties, curing mechanisms, and commercial forms of acrylic resin is essential to selecting the right material for each case, whether traditional or digitally produced. By embracing modern acrylic systems and digital workflows, labs can achieve higher accuracy, consistency, and long-term satisfaction for both clinicians and patients.
At XDENT LAB, we believe that combining scientific knowledge, digital craftsmanship, and material innovation is the key to shaping the next generation of prosthetic dentistry, where every restoration delivers durability, comfort, and natural beauty.
References
Characteristics of Acrylic Produced Additively by 3D Printing in Dentistry: Comparison of Mechanical and Surface Parameters—A Systematic Review with Meta-Analysis of Novel Reports written by Szymlet et al. 2025.
5 - Acrylic denture base materials written by Nejatian et al. 2019.
Compressive Strength of Three Types of Heat-Cure Acrylic Resins: Acropars, Acrosun, and Meliodent written by Neshati et al. 2021.
Update on Acrylic Resins used in Dentistry written by Raszewski et al. 2021.
XDENT LAB is an expert in Lab-to-Lab Full Service from Vietnam, with the signature services of Removable & Implant, meeting U.S. market standards – approved by FDA & ISO. Founded in 2017, XDENT LAB has grown from local root to global reach, scaling with 2 factories and over 100 employees.. Our state-of-the-art technology, certified technicians, and commitment to compliance make us the trusted choice for dental practices looking to ensure quality and consistency in their products.
Our commitments are:
100% FDA-Approved Materials.
Large-Scale Manufacturing, high volume, remake rate < 1%.
2~3 days in lab (*digital file).
Your cost savings 30%.
Uninterrupted Manufacturing 365 days a year.
Contact us today to establish a strategy to reduce operating costs.
--------❃--------
Vietnam Dental Laboratory - XDENT LAB
🏢 Factory 1: 95/6 Tran Van Kieu Street, Binh Phu Ward, Ho Chi Minh City, Vietnam
🏢 Factory 2: Kizuna 3 Industrial Park, Can Giuoc Commune, Tay Ninh Province, Vietnam
☎ Hotline: 0919 796 718 📰 Get detailed pricing
Share this post: