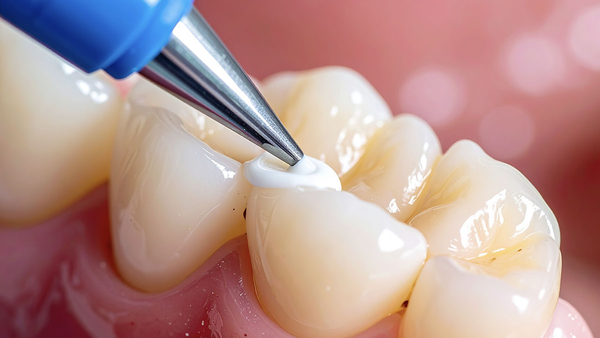
An academic review of unfilled resin sealants covering composition, low‑viscosity penetration, enamel tag formation, retention rates, and evidence‑based protocols.
Table of contents [Show]
- Overview
- Introduction & Definition
- Chemical Composition & Properties
- Penetration Ability & Mechanics
- Clinical Advantages
- Comparative Analysis: Unfilled vs Filled Sealants
- Specific Clinical Indications
- Application Technique Optimization
- Quality Control & Evaluation
- Material Science Considerations
- Research Evidence & Clinical Studies
- Special Considerations
- Future Developments
- Best‑Practice Recommendations
- Practice‑Level Takeaways
Overview
Unfilled resin sealants are integral to preventive dentistry. By removing inorganic fillers, these materials achieve low viscosity and superior fissure penetration—translating into strong early retention and predictable chairside outcomes. Below is a comprehensive review of their composition, mechanics, clinical performance, indications, and best‑practice protocols, geared for clinicians and practice leaders seeking standardized, high‑quality preventive care.
Introduction & Definition
Unfilled resin sealants are pit and fissure sealants formulated without inorganic filler particles. The absence of fillers reduces viscosity, enhances flow, and improves wetting of etched enamel, enabling deeper penetration into narrow and complex fissures. Across systematic reviews and meta‑analyses, unfilled sealants demonstrate superior early retention and comparable caries prevention to filled sealants when retained—underscoring their continued clinical relevance, particularly in pediatric and high‑risk populations.
Chemical Composition & Properties
This section outlines typical formulation ranges and the handling traits clinicians can expect.
Basic Composition
Resin matrix (95–99%):
BIS‑GMA: 40–60%.
TEGDMA: 30–50%.
UDMA: 0–20%.
Other dimethacrylates: 5–10%.
Polymerization system (1–5%):
Photoinitiators (camphorquinone): 0.2–1%.
Co‑initiators (tertiary amines): 0.1–0.5%.
Stabilizers/inhibitors: 0.1–0.3%.
UV absorbers: trace.
Physical Characteristics
Viscosity: 0.5–2.0 Pa·s; excellent flow; minimal thixotropy.
Optical: clear to slightly amber; high transparency (>90%); refractive index 1.48–1.52.
Handling: very fluid; moderate temperature sensitivity; self‑leveling behavior.
Clinical implication: low viscosity enhances capillary action and reduces bubble entrapment, supporting reliable penetration and microretention.
Penetration Ability & Mechanics
Unfilled resins consistently show superior penetration and lateral wall adaptation in deep, narrow fissures.
Penetration Characteristics
Penetration depth: 90–100% of fissure depth.
Microporosities filled: >95%.
Contact angle: <20°, indicating strong wetting.
Bubble entrapment: minimal with proper isolation.
Mechanism of Action
Physical processes:
Initial flow into the fissure entrance.
Capillary action draws resin deeper.
Air displacement facilitated by low viscosity.
Lateral spread and wall adaptation.
Conformity to irregularities and undercuts.
Chemical interactions:
Enamel tag formation: ~10–40 μm.
Hybrid layer: ~2–5 μm.
Primary retention via mechanical interlocking (microretention).
Clinical implication: deeper and denser resin tags support higher early retention and stable margins in preventive applications.
Clinical Advantages
Lower viscosity yields practical gains in retention and workflow efficiency.
Retention Mechanisms
Deeper enamel tag penetration.
Higher tag density across etched enamel.
Superior adaptation to fissure morphology and undercuts.
Increased contact surface area for microretention.
Performance Benchmarks
1‑year retention: 85–95%.
3‑year retention: 70–85%.
5‑year retention: 55–70%.
Early complete retention rates higher than filled sealants; partial losses often less detrimental to caries prevention.
Application Benefits
Easier, faster placement with self‑leveling.
Better visibility during placement.
Reduced air entrapment.
Lower remake rates and chair time.
Predictable outcomes suitable for standardized protocols.
Comparative Analysis: Unfilled vs Filled Sealants
Understanding trade‑offs helps direct material selection to case needs.
Physical Property Differences
Viscosity:
Unfilled: 0.5–2.0 Pa·s.
Filled: 3.0–10.0 Pa·s.
Mechanical properties:
Flexural strength: 60–80 MPa (unfilled) vs 80–120 MPa (filled).
Hardness: 15–25 KHN (unfilled) vs 25–40 KHN (filled).
Wear resistance and fracture toughness: lower for unfilled.
Clinical Performance Comparison
Retention (typical across studies):
6 months: Unfilled ~92% vs Filled ~88%.
12 months: Unfilled ~87% vs Filled ~83%.
24 months: Unfilled ~78% vs Filled ~75%.
36 months: Unfilled ~71% vs Filled ~69%.
Caries prevention:
Both effective when retained.
Marginal integrity initially better with unfilled.
Long‑term effectiveness similar; cost‑effectiveness comparable.
Practical positioning: prefer unfilled for deep, narrow fissures, pediatric, and preventive programs; consider filled for high‑stress occlusal surfaces where wear resistance is critical.
Specific Clinical Indications
Targeted use maximizes clinical benefit and operational efficiency.
Ideal Applications
Deep narrow fissures: complete depth sealing; reduced voids; superior adaptation.
Primary teeth: smaller fissures; shorter service time; simpler pediatric workflow.
Preventive programs: school‑based/high‑volume settings; simplified technique; reduced equipment needs.
Contraindications & Limitations
Relative contraindications: high occlusal stress, bruxism, extensive occlusal surfaces, previously restored teeth, poor moisture control.
Limitations: lower wear resistance; reduced radiopacity; monitoring challenges; potential discoloration.
Application Technique Optimization
Protocol precision improves retention and reduces remakes.
Surface Preparation
Cleaning: fluoride‑free pumice, thorough rinse, explorer debris removal, complete dry, visual inspection.
Etching: 35–37% phosphoric acid for 15–20 seconds; extend 2–3 mm beyond fissures; rinse equal to etch time; oil‑free air dry.
Sealant Application
Steps:
Dispense single drop.
Place at the deepest fissure first.
Allow 15–20 seconds undisturbed flow.
Optional gentle air‑thinning for excess.
Bubble removal with explorer if needed.
Critical factors: avoid overfilling; allow natural flow; maintain a dry field; verify complete coverage.
Polymerization Protocol
Light curing: 470 nm; ≥400 mW/cm²; <2 mm distance; ≥20 seconds; overlap for large surfaces.
Post‑cure: remove oxygen‑inhibited layer; adjust occlusion if necessary; explorer retention check; optional fluoride application.
Quality Control & Evaluation
Standardized QA lowers variability and supports consistent outcomes.
Immediate Assessment
Visual: full fissure coverage; no bubbles; smooth surface; appropriate thickness; intact margins.
Tactile: explorer retention; marginal adaptation; smoothness; absence of tackiness; complete cure.
Long‑Term Monitoring
Follow‑up schedule: 3 months → 6 months → 12 months → risk‑based intervals.
Evaluation criteria: retention status (complete/partial/lost), marginal integrity, surface characteristics, caries presence, reapplication needs.
Documentation: standardized forms, photo records where appropriate.
Material Science Considerations
Chairside behavior tracks with polymerization kinetics and aging dynamics.
Polymerization Kinetics
Degree of conversion: surface ~65–75%; depth ~55–65%.
Influences: light intensity, exposure time, distance.
Shrinkage and stress:
Volume shrinkage: 3–5%.
Linear shrinkage: 1–2%.
Lower stress vs filled sealants; flow aids margin compensation.
Aging & Degradation
Water sorption: 1–3% wt; solubility <0.5%.
Stability: minimal thermal cycling effects; good pH stability; moderate enzymatic resistance.
Clinical: surface wear primary; progressive marginal breakdown; rare bulk fracture; possible discoloration; retention loss over time.
Research Evidence & Clinical Studies
Evidence converges on strong early retention and equivalent caries prevention under retention.
Early retention superiority for unfilled sealants.
Equivalent caries prevention when retained.
Slight cost‑effectiveness advantage and lower technique sensitivity.
Clinical Trials
Penetration depth: 25–40% greater with unfilled.
Initial retention: 5–10% higher.
Faster application (20–30%); higher patient preference.
Laboratory Studies
SEM: superior wall adaptation.
Micro‑CT: complete fissure penetration.
Confocal microscopy: enhanced resin tag formation.
Cross‑sectional analysis: void‑free interfaces.
Special Considerations
Unfilled sealants fit best where fast, predictable workflows are essential.
Pediatric Applications
Faster application; clear appearance; reduced chair time.
Better flow in small teeth; easier isolation; predictable outcomes at lower cost.
High‑Risk Populations
Suitable for medically compromised, xerostomic, orthodontic, geriatric, and limited‑cooperation patients due to shorter protocols and robust early retention.
Future Developments
Innovation aims to preserve flow advantages while enhancing durability and therapeutic function.
Material Innovations
Fluoride‑releasing unfilled resins.
Self‑adhesive formulations.
Antimicrobial additives.
Color‑change indicators.
Smart release systems.
Clinical Applications
Adult preventive care expansion.
Sealing non‑cavitated lesions.
Orthodontic applications around brackets.
Hypersensitivity management.
Therapeutic sealing strategies.
Best‑Practice Recommendations
Evidence‑driven guidance for daily clinical practice and program standardization.
Clinical Guidelines
Prefer unfilled sealants for deep, narrow fissures and pediatric/high‑risk cases.
Maintain strict isolation and moisture control.
Allow adequate undisturbed flow time.
Avoid overfilling; leverage self‑leveling behavior.
Monitor retention at defined intervals and reapply when needed.
Evidence‑Based Protocol
Thorough cleaning and drying.
Phosphoric acid etch 15–20 seconds; extend coverage; equal rinse time.
Complete isolation; oil‑free air drying.
Controlled dispensing; deepest fissure first; bubble management.
Proper light curing; overlap on large surfaces.
Immediate QA (visual/tactile); occlusal adjustment as needed.
Scheduled follow‑ups; standardized documentation.
Practice‑Level Takeaways
Unfilled resin sealants deliver superior early retention and predictable workflows—ideal for pediatric and preventive programs. The trade‑off is lower wear resistance compared to filled sealants; reserve filled materials for high‑stress occlusion. Standardized protocols and QA reduce variability and remakes, supporting consistent outcomes across providers and sites.
For multi‑site practices implementing standardized preventive care, FDA/ISO‑aligned QC systems and documented protocols help ensure repeatable results. XDENT LAB’s lab‑to‑lab services support consistency through protocol training, traceable materials, and performance monitoring designed to meet U.S. standards.
XDENT LAB is an expert in Lab-to-Lab Full Service from Vietnam, with the signature services of Removable & Implant, meeting U.S. market standards – approved by FDA & ISO. Founded in 2017, XDENT LAB has grown from local root to global reach, scaling with 2 factories and over 100 employees.. Our state-of-the-art technology, certified technicians, and commitment to compliance make us the trusted choice for dental practices looking to ensure quality and consistency in their products.
Our commitments are:
100% FDA-Approved Materials.
Large-Scale Manufacturing, high volume, remake rate < 1%.
2~3 days in lab (*digital file).
Your cost savings 30%.
Uninterrupted Manufacturing 365 days a year.
Contact us today to establish a strategy to reduce operating costs.
--------❃--------
Vietnam Dental Laboratory - XDENT LAB
🏢 Factory 1: 95/6 Tran Van Kieu Street, Binh Phu Ward, Ho Chi Minh City, Vietnam
🏢 Factory 2: Kizuna 3 Industrial Park, Can Giuoc Commune, Tay Ninh Province, Vietnam
☎ Hotline: 0919 796 718 📰 Get detailed pricing
Share this post: