This article gives an insight on the importance of disinfection of dental impressions in preventing cross contamination and also emphasizes the various disinfection modalities recommended for various impression materials.

Table of contents [Show]
Key Points
Dental impressions are a high-risk but highly preventable source of cross-contamination in dental labs.
Rinsing isn’t enough: water alone removes only part of the microbial load; disinfection is non-negotiable.
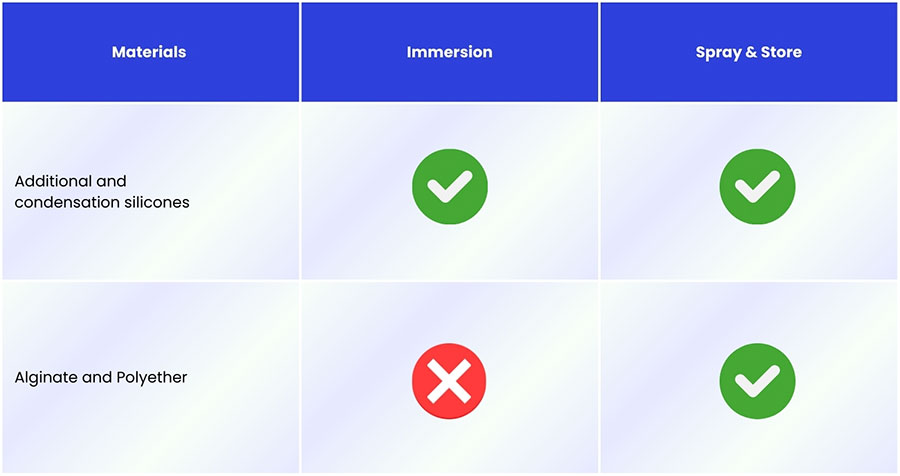
Disinfection must be material-driven: silicones tolerate immersion; alginate and polyether should be spray-only to avoid distortion.
Labs need to choose the right mix of chemical and physical methods based on safety + dimensional stability.
A step-by-step cleaning and disinfection workflow is now a core quality and biosafety benchmark for dental laboratories.
Introduction
Dentistry constantly exposes people and materials to saliva, blood, and other potentially infectious fluids. Just 1 ml of saliva from a healthy person can contain around 750 million microorganisms, and roughly 280 bacterial species have been isolated from the oral cavity. In patients wearing prosthetic or removable orthodontic appliances, common colonizers include Staphylococcus, Streptococcus, Lactobacillus, Actinomyces, and Candida species.
This microbial burden makes oral health-care professionals and dental technicians particularly vulnerable to cross-infection, as dental materials often go directly from the patient’s mouth to the dental laboratory. A review by Chidambaranathan AS et al. (2019) reported that 67% of items received in laboratories were contaminated with organisms such as Streptococci, Staphylococci, Candida spp., methicillin-resistant S. aureus (MRSA), and P. aeruginosa. In addition, another study said that dental personnel are estimated to have a 5 –10-fold higher risk of acquiring Hepatitis B than the general population, and both Tuberculosis and Hepatitis B pathogens can survive for seven days or longer at room temperature.
For lab owners and dental technicians, these realities highlight that infection control and standardized disinfection of dental materials are not optional; they are fundamental to preventing cross-contamination and ensuring a safe laboratory environment.
Understanding Cross-Contamination Risks in Dental Laboratories
Cross-contamination in dental environments can occur through multiple interconnected pathways, including:
Between patients
From patients to dental professionals
From dental professionals back to patients
Between clinical and laboratory teams, especially through shared tools, impression materials, prostheses, and transport systems
From laboratory personnel to dentists and subsequently to other patients, when contaminated casts or prosthetic components are handled and returned to the clinic
In the laboratory setting, this risk becomes even more pronounced. When technicians work on contaminated impressions, casts, or prosthetic components, microorganisms from one patient’s case can easily be transferred to another. Over time, this contamination can travel full circle: from the patient → to the dentist → to the laboratory → back to the dentist → and ultimately to new patients.
For dental labs, where multiple cases are processed simultaneously and materials frequently move between workstations, cross-contamination is not an isolated event but a systemic vulnerability.
Dental Impression – A Possible source of cross-contamination
As we map out complex transmission pathways, one category of material consistently emerges as a critical control point in every dental laboratory workflow: the dental impression.
Impression making is a fundamental part of dental treatment, used to create a replica of the oral structures. During this procedure, impression materials are heavily exposed to saliva and blood, which may contain infectious agents such as HIV, herpes, hepatitis, or tuberculosis. If not properly managed, these contaminated impressions can transfer microorganisms to stone casts and become a direct source of infection for anyone handling them.
Rinsing impressions under running water is a helpful first step, but studies show it removes only about 40% of microorganisms. Therefore, proper disinfection is mandatory, not optional. Today, several methods are used to disinfect impressions and models, including chemical disinfection (immersion/spray), microwave, autoclave, and ultraviolet (UV) radiation, which we will examine in the next section.
Disinfection Methods
Chemical disinfection methods
For most dental labs, chemical disinfection is the primary approach. When the material allows, immersion is generally the most reliable option because all impression surfaces are fully exposed to the disinfectant solution. This works well for addition and condensation silicones, which are dimensionally stable. However, immersion is not suitable for hydrophilic materials such as alginate and polyether, as they can absorb the solution and distort. It is also more time-consuming and most solutions must be discarded after each use.
In these cases, the spray & store technique is preferred. Spraying reduces the risk of dimensional change, especially for alginate and polyether, but may be less effective in deep undercuts because a smaller volume of disinfectant is used and full coverage is harder to guarantee.
This material-dependent choice between immersion and spray & store is summarized in Figure 1.
Glutaraldehyde (2%)
Disinfection Level: High
Overview: Glutaraldehyde is highly effective and capable of destroying a broad range of microorganisms, including bacteria, viruses, tuberculosis bacilli, and even sporulated fungi, when used correctly.
Advantages
Provides high-level disinfection
Rapid antimicrobial action
Effective against a wide spectrum of pathogens
Limitations
Contraindicated for routine use due to significant health risks
Strong odor; can irritate eyes, skin, and respiratory tract
Requires handling in closed containers and well-ventilated areas
Not biodegradable → banned in several countries
Must be handled with nitrile gloves and strict PPE
Sodium Hypochlorite
Disinfection level: Intermediate
Overview: Sodium hypochlorite is a widely used disinfectant with a broad spectrum of antimicrobial activity. It acts mainly through oxidation, which makes it highly effective against many viruses, including COVID-19. Studies have shown that 1% sodium hypochlorite spray on alginate impressions (after rinsing and drying) does not cause severe dimensional changes, although prolonged immersion (e.g., 15 minutes in 0.5%) can lead to slight dimensional alterations.
Advantages
Broad antimicrobial spectrum
Fast antimicrobial action
Easy to use and water-soluble
Relatively stable at recommended concentrations
Non-toxic at indicated concentration
Low cost
Does not stain materials
Non-flammable and colorless
Limitations
Irritates mucous membranes
Less effective in the presence of organic matter
Corrosive to metals
Prolonged immersion may cause slight dimensional changes in some impression materials
Iodoform
Disinfection level: Low to intermediate
Overview: Iodoform provides bactericidal, mycobactericidal, and virucidal activity, with some fungicidal effect, although fungi require longer contact time. It is generally better suited as an antiseptic rather than a primary impression disinfectant. One study reported that 30 minutes of exposure to 0.1% povidone-iodine did not cause significant distortion in polysulfide or polyvinyl siloxane impressions.
Advantages
Broad antimicrobial activity (bacteria, mycobacteria, viruses, fungi)
Non-flammable
Can be used on certain elastomeric impression materials without major distortion
Limitations
Not sporicidal
Can cause staining
May irritate membranes and mucous tissues
Effectiveness decreases in the presence of organic debris
Requires prolonged contact time for reliable disinfection
Alcohol
Disinfection level: Intermediate
Overview: Alcohol-based solutions, typically 70% ethyl or isopropyl alcohol, provide intermediate disinfection and are widely used as antiseptics and for surface cleaning. Ethyl alcohol has stronger bactericidal activity, while isopropyl alcohol is commonly used for general antiseptic purposes. Both are effective against tuberculosis bacilli, fungi, and many viruses.
Advantages
Fast-acting antimicrobial activity
Widely available and easy to use
Effective against a broad range of microorganisms
Suitable for disinfecting office surfaces and non-porous equipment
Limitations
Not recommended for impression disinfection due to surface alteration
Can damage or distort impression materials
Not suitable for disinfecting acrylic prosthesis bases
Flammable and requires proper handling
Phenols
Disinfection level: Intermediate
Overview: Phenolic compounds act as protoplasmic poisons, disrupting microbial cell walls and promoting lysis. Even at low concentrations, they are effective against E. coli, Staphylococcus, and Streptococcus, and also possess antifungal and antiviral properties. Phenols are commonly used in mouthwashes, soaps, and for general surface cleaning.
Advantages
Effective against a wide range of bacteria
Antifungal and antiviral properties
Useful for surface cleaning applications
Limitations
Not suitable for disinfecting impressions
Incompatible with latex, acrylic, and rubber
May cause material degradation or distortion
Chlorhexidine
Disinfection level: Intermediate
Overview: Chlorhexidine is a widely used disinfectant and antiseptic with a broad antimicrobial spectrum. It is commonly found in oral rinses and cleansing solutions. Chlorhexidine is bactericidal, virucidal, and mycobacteriostatic, though its effectiveness decreases in the presence of organic material due to its pH-dependent activity. Studies show that 0.2% chlorhexidine can replace water when preparing alginate, and impressions can also be immersed in chlorhexidine to achieve effective disinfection.
Advantages
Broad antimicrobial spectrum
Suitable for immersion disinfection of certain impression materials
Can be used as mixing liquid for alginate
Ideal for disinfecting prostheses with metal components, where hypochlorite is contraindicated
Limitations
Reduced activity in the presence of organic debris
pH-dependent effectiveness
May be less effective on heavily contaminated impressions without prior cleaning
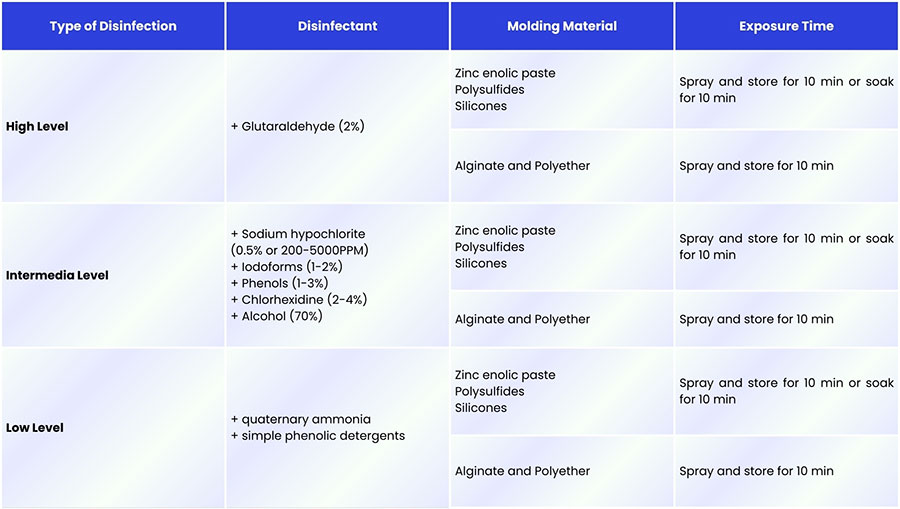
Levels of Disinfection With Chemical Disinfectants
Chemical disinfectants are generally classified into three levels based on their effectiveness against vegetative bacteria, tubercle bacilli, fungal spores, and viruses:
High-level disinfection: Inactivates most pathogenic microorganisms.
Intermediate-level disinfection: Effective against organisms such as Mycobacterium tuberculosis, but does not reliably destroy spores.
Low-level disinfection: Provides limited antimicrobial activity, targeting only some bacteria and viruses.
The appropriate disinfection level depends on both the disinfecting agent and the impression material being treated. The compatibility between materials and disinfectant types is summarized in Figure 2, showing which agents can be used safely without compromising dimensional accuracy.
Microwave disinfection methods
Disinfection level: Effective physical disinfection method
Overview: Microwave disinfection is a quick, simple, and inexpensive method suitable for dentists, assistants, and dental technicians. Microwave disinfection disrupts microbial cell membranes and interferes with cell metabolism, resulting in effective disinfection.
Advantages
Fast and easy to perform
Cost-effective compared to many chemical agents
Does not require specialized chemical handling
Suitable for dental offices and laboratories
Limitations
Not all impression materials are microwave-compatible
Risk of deformation or overheating of certain materials
Requires correct power and exposure settings to avoid damage
Autoclave disinfection methods
Steam autoclave
Overview: Steam autoclave is a device used to sterilize equipment, surgical instruments in medicine and dentistry by subjecting them to high saturated steam pressure at 121°C or more for 15 to 20 minutes.
Advantages
Provides true sterilization, including spores
Rapid cycle at 134°C (as short as ~3 minutes)
Compatible with certain addition and condensation silicone impression materials
Widely available in dental clinics and hospitals
Limitations
Not suitable for all tray types or impression materials
Risk of distortion if materials or custom trays are not heat-stable
Requires correct loading and cycle parameters to avoid deformation
Ethylene oxide gas autoclave
Overview: Ethylene oxide gas has been investigated as another method for disinfecting or sterilizing impressions. Studies (Holtan et al., 1991; Olin et al., 1994) reported that gas sterilization of vinyl polysiloxane (VPS) impressions can lead to gas inclusion within the material, which may later be released and cause bubbles in stone dies, especially if poured immediately.
Advantages
Capable of low-temperature sterilization
Useful in theory for heat-sensitive devices
Limitations
Gas inclusion in VPS can produce bubbles in dies if poured too soon (dies should be delayed ~24 hours)
Reports of significant structural changes (>0.5% dimensional change) in some silicone impressions and trays
Longer cycle times and more complex handling than steam autoclaves
Less practical and less commonly used for routine impression disinfection in dental labs
Ultra-Violet Radiation
Disinfection level: Intermediate to high (dependent on intensity, exposure time, and accessibility)
Overview: Ultraviolet (UV) light can serve as an effective disinfectant, but its efficiency depends heavily on exposure time, light intensity, humidity, and the ability of UV rays to reach microbial surfaces. Because dental impressions and prostheses contain complex surface details that can shelter microorganisms, UV light must be delivered from multiple angles to achieve meaningful microbial reduction.
Studies have shown that UV exposure can significantly reduce C. albicans colonies, with higher-wattage UV tubes producing faster and more complete microbial elimination. Maximum killing efficiency has been observed at 24 watts (3750 μw/cm²). Samra et al. (2018) also reported that UV disinfection is suitable for impressions without compromising dimensional stability.
Advantages
Effective against fungi such as C. albicans
Higher wattage results in faster microbial reduction
Does not involve moisture or chemicals → minimal risk of dimensional distortion
Suitable for impressions where material stability is a concern
Limitations
Effectiveness depends on complete light access to all impression surfaces
Complex surface undercuts may limit full disinfection
Performance influenced by environmental conditions (humidity, intensity)
Requires appropriate equipment and safety shielding to prevent UV exposure to personnel
Step-by-Step Cleaning and Disinfection of Dental Impressions
While each disinfection method offers its own advantages and limitations, none of them can be effective without proper pre-cleaning. Residual blood, saliva, and organic debris can significantly reduce the antimicrobial efficacy of chemical, thermal, or UV-based approaches. For this reason, industry best practices emphasize that cleaning is the mandatory first step before any disinfection protocol is applied. To ensure both biosafety and impression accuracy, the following step-by-step cleaning procedure should be consistently followed in every dental laboratory workflow.
Effective disinfection begins with a proper cleaning process. Removing saliva, blood, and organic debris is essential because residual contamination can reduce the efficacy of any chemical, microwave, autoclave, or UV-based disinfection method. The following steps outline the recommended cleaning protocol for dental impressions:
1. Rinse Under Running Water
Immediately after removal from the patient’s mouth, the impression should be thoroughly rinsed under running water to remove visible contaminants such as blood, saliva, and debris.
Do not use air or steam for drying, as this may create aerosols and increase biological risk.
Allow water to drain naturally in the same area to reduce splash dispersion.
Techniques such as air-blowing may spread microorganisms into the environment. Maintaining a low-aerosol workflow is critical for technician safety.
3. Prepare the Impression for Disinfection
Shake off excess water gently. The surface should remain moist, as drying may interfere with the action of certain disinfectants, especially when using spray methods.
4. Apply the Appropriate Disinfection Method
Based on the impression material (e.g., silicone vs. alginate), choose the correct method:
Immersion for dimensionally stable materials like addition or condensation silicone
Spray & store for hydrophilic materials such as alginate or polyether
Thermal/physical methods (microwave, autoclave, UV) when compatible
5. Final Rinse
After the recommended contact time with the disinfectant, rinse the impression again under running water.
6. Dry and Proceed to Casting
Gently shake off excess water and allow the impression to dry in a clean area before pouring. Avoid using heat or airflow that could distort the material.
Conclusion
Cross-contamination remains a significant concern in dental laboratories, and impressions represent one of its most common, and preventable sources. By understanding material-specific risks and applying validated disinfection methods, labs can protect both technicians and patients while maintaining the dimensional accuracy essential for high-quality prosthetic outcomes. For modern dental laboratories, consistent cleaning, proper disinfection, and well-designed workflows are not just safety measures, they are essential foundations for reliable, efficient, and trustworthy laboratory practice.
References
Disinfection of Impression Materials: A Comprehensive Review of Disinfection
XDENT LAB is an expert in Lab-to-Lab Full Service from Vietnam, with the signature services of Removable & Implant, meeting U.S. market standards – approved by FDA & ISO. Founded in 2017, XDENT LAB has grown from local root to global reach, scaling with 2 factories and over 100 employees.. Our state-of-the-art technology, certified technicians, and commitment to compliance make us the trusted choice for dental practices looking to ensure quality and consistency in their products.

Our commitments are:
100% FDA-Approved Materials.
Large-Scale Manufacturing, high volume, remake rate < 1%.
2~3 days in lab (*digital file).
Your cost savings 30%.
Uninterrupted Manufacturing 365 days a year.
Contact us today to establish a strategy to reduce operating costs.
--------❃--------
Vietnam Dental Laboratory - XDENT LAB
🏢 Factory 1: 95/6 Tran Van Kieu Street, Binh Phu Ward, Ho Chi Minh City, Vietnam
🏢 Factory 2: Kizuna 3 Industrial Park, Can Giuoc Commune, Tay Ninh Province, Vietnam
☎ Hotline: 0919 796 718 📰 Get detailed pricing
Share this post: