Dental FDM explained: study models, surgical planning, education, parameters, and costs. Practical guidance with ISO‑aligned quality controls.
Table of contents [Show]
- Overview
- Technical Overview of Dental FDM
- Clinical Applications in Dentistry
- Educational & Communication Value
- Parameters & Quality for Dental Prints
- FDM vs. Other Dental 3D Printing
- Regulatory & Safety Considerations
- Economics for Practices & Labs
- Limitations & Challenges
- Future Directions in Dental FDM
- Best Practices for Implementation
- Practical Takeaways for Dental Teams
Overview
Fused Deposition Modeling (FDM) has earned a pragmatic place in digital dentistry thanks to its low cost, simple operation, and rapid turnaround. While it cannot match resin-based systems (SLA/DLP) for precision intraoral devices, FDM excels for study models, educational replicas, pilot surgical guides, and communication tools - Making it a smart entry point and a complementary technology in a multi-printer environment.
Technical Overview of Dental FDM
FDM in dentistry mirrors general FDM mechanics but adapts to dental imaging and CAD/CAM workflows.
Dental-Specific Workflow
Digital acquisition: intraoral scans/CBCT.
CAD: tooth libraries, occlusion setup, surgical planning.
STL preparation: watertight mesh, shelling, supports.
Slicing: dental-tuned parameters and orientations.
Printing: layer-by-layer thermoplastic deposition.
Post-processing: support removal, smoothing, sterilization (if required).
Materials for Dental Use
PLA: stable, low-warp models; suitable for education and records.
PETG: tougher, chemical-resistant models; useful for splint patterns.
ABS: durable; vapor-smoothable; pilot surgical guides (non-implant-contact).
TPU: flexible models and protectors (non-intraoral use).
PC/PC-blends: higher heat resistance; select sterilization compatibility.
Key properties needed: Biocompatibility for intended use (most FDM parts are non-patient-contact), sterilization compatibility where applicable, and dimensional/color stability for reliable communication and records.
Clinical Applications in Dentistry
FDM’s strength is fast, cost-effective models and planning aids rather than final intraoral devices.
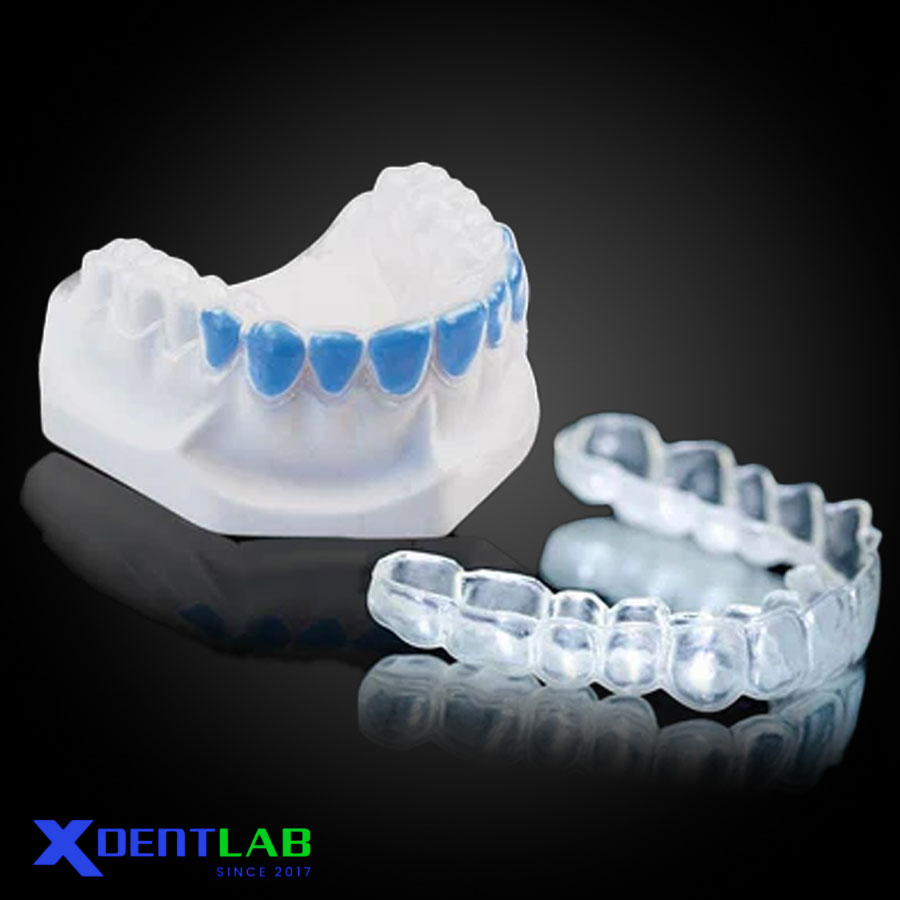
Orthodontics
Study models for records, planning, progress, and case presentation.
Temporary retainers/splint patterns and emergency backups.
Bracket positioning (indirect bonding) pilot guides for pre-visualization.
Surgery & Planning
Pilot implant/osteotomy guides for planning (not for final placement).
Resection/reconstruction planning models.
Anatomical replicas for team rehearsal and patient consent.
Advantages: low-cost iteration, in-house control, fast refinements.
Prosthodontics
Diagnostic wax-up visualizations.
Try-in denture bases and occlusion checking models.
Custom tray and splint patterns (printed patterns for subsequent fabrication).
Master casts for communication and mounting (non-definitive).
Educational & Communication Value
Dental education: anatomy, pathology, and procedure simulation models.
Student skill practice: cavity prep, access, crown prep on printed typodonts.
Patient education: demonstrate tooth movement, implant positioning, surgery steps.
Research: repeatable models for method comparisons and device testing.
Parameters & Quality for Dental Prints
Balancing accuracy, speed, and cost is essential for predictable outcomes.
Recommended Ranges (Models & Guides)
Layer height: 0.1–0.2 mm for improved detail without large time penalties.
Infill: 40–60% (grid/honeycomb) for stiffness and efficiency.
Speed: 40–60 mm/s for surface quality and dimensional stability.
Temperatures: material-specific; keep filament dry to prevent porosity.
Supports: minimize via orientation; use breakaway or soluble for fine features.
Accuracy Targets
Dimensional tolerance: ±0.3–0.5 mm typical for desktop FDM.
Feature resolution: ≥0.4 mm (0.4 mm nozzle baseline).
Occlusal detail: acceptable for planning and communication, not for definitive margins.
Conclusion: suitable for study models and planning; not for final restorations.
Post-Processing
Support removal with detail preservation.
Mechanical finishing, media tumbling, or vapor smoothing (ABS).
Cleaning and optional sealing coats for aesthetics and durability.
Sterilization only when material and geometry permit (verify deformation risk).
FDM vs. Other Dental 3D Printing
FDM complements resin and powder-bed systems rather than replaces them.
Comparative Summary
FDM: Accuracy ±0.3–0.5 mm; lowest cost and easy operation; best for study models, education, pilot guides; caveats: layer lines, limited fine detail.
SLA/DLP: Accuracy ±0.05–0.1 mm; moderate cost; best for splints, trays, precise guides, provisionals; caveats: resin handling and post-cure.
SLS/MJF: Accuracy ±0.1–0.2 mm; high cost; best for durable nylon parts and frameworks; often outsourced.
PolyJet: Accuracy ±0.05–0.1 mm; high cost; best for multi-material demos; expensive materials/maintenance.
Decision factors: accuracy needs, material requirements, production volume, budget, and in-house capability.
Regulatory & Safety Considerations
Intended use:
Educational models: generally Class I and often exempt.
Surgical guides contacting tissue/bone: typically Class II (510(k) for U.S.) when used clinically; FDM guides commonly for planning, not placement.
Quality management: adopt ISO 13485–aligned procedures if producing devices.
Materials: confirm biocompatibility for any patient-contact device.
Safety: MSDS, ventilation for ABS/PC, PPE, waste handling, cross-contamination control.
Economics for Practices & Labs
Printer costs: $500–$5,000; filament: $20–$50/kg; low maintenance and energy.
Savings: 60–80% vs. outsourced study models; 24–48 h turnaround in-house.
ROI: rapid when used for orthodontic models, consult visuals, and surgical planning.
Strategic value: improved case acceptance and communication; lab–clinic alignment.
Limitations & Challenges
Accuracy/finish: visible layers, limited margin fidelity, rough surfaces.
Materials: limited directly intraoral-safe options; heat/sterilization constraints.
Mechanical anisotropy: orientation affects strength and detail.
Skill: parameter tuning and post-processing significantly impact outcomes.
Future Directions in Dental FDM
Higher-resolution motion systems and nozzles; non-planar slicing to reduce staircase effect.
Multi-material deposition for support and flexible features.
AI-driven slicing and closed-loop monitoring for dimensional control.
New filaments: antimicrobial, biocompatible grades, and better sterilization resistance.
Deeper integration with scanner-to-printer cloud workflows and automated QA.
Best Practices for Implementation

Equipment & Environment
Enclosed chamber, reliable bed leveling, dual extrusion (for soluble supports).
Dry-box for hygroscopic filaments (e.g., PA, TPU), HEPA/charcoal filtration for ABS/PC.
Workflow & Quality Control
Standardized STL prep and parameter libraries by indication.
Calibration coupons and first-article checks.
Dimensional verification at key landmarks, batch tracking, documented parameters.
Scheduled maintenance and operator training on slicing, supports, and material handling.
Retain scan-to-print traceability and QA records for audits and lab–clinic communication.
Practical Takeaways for Dental Teams
Use FDM where it excels: study models, planning aids, educational pieces, pilot guides.
For high-precision intraoral devices, combine FDM with SLA/DLP and validated resins.
Stabilize environment and materials (dry filament, enclosed chamber) to reduce warping and variability.
Treat FDM as part of a compliant digital workflow with documented parameters and QC checks.
XDENT LAB integrates FDM alongside resin workflows to deliver fast, reliable models and planning aids within FDA/ISO-aligned processes. This ensures traceable materials, validated parameters, and consistent outcomes - Giving clinicians clear communication tools and labs predictable production without compromising regulatory expectations.
XDENT LAB is an expert in Lab-to-Lab Full Service from Vietnam, with the signature services of Removable & Implant, meeting U.S. market standards – approved by FDA & ISO. Founded in 2017, XDENT LAB has grown from local root to global reach, scaling with 2 factories and over 100 employees.. Our state-of-the-art technology, certified technicians, and commitment to compliance make us the trusted choice for dental practices looking to ensure quality and consistency in their products.

Our commitments are:
100% FDA-Approved Materials.
Large-Scale Manufacturing, high volume, remake rate < 1%.
2~3 days in lab (*digital file).
Your cost savings 30%.
Uninterrupted Manufacturing 365 days a year.
Contact us today to establish a strategy to reduce operating costs.
--------❃--------
Vietnam Dental Laboratory - XDENT LAB
🏢 Factory 1: 95/6 Tran Van Kieu Street, Binh Phu Ward, Ho Chi Minh City, Vietnam
🏢 Factory 2: Kizuna 3 Industrial Park, Can Giuoc Commune, Tay Ninh Province, Vietnam
☎ Hotline: 0919 796 718 📰 Get detailed pricing
Share this post: